Hg/Hg2SO4 Reference Electrode
The Hg/Hg2SO4 reference electrode is a specialized electrochemical solution for chloride-free applications with acidic stability. This mercury/mercurous sulfate electrode provides reliable measurements for research/industrial use.
Product Descriptio
Core Design Elements
The electrode features a glass body construction with a microporous ceramic filter junction that ensures steady electrolyte flow while maintaining measurement integrity. The internal system consists of metallic mercury in contact with mercurous sulfate (Hg2SO4), creating the fundamental electrochemical reaction:
Hg2SO4(s) + 2e⁻ ⟷ 2Hg(l) + SO4²⁻
This reaction establishes a standard electrode potential of +0.615 to +0.674 V versus Normal Hydrogen Electrode (NHE) at 25°C, with reported values ranging from +0.62 to +0.66 V depending on the specific sulfate electrolyte concentration.
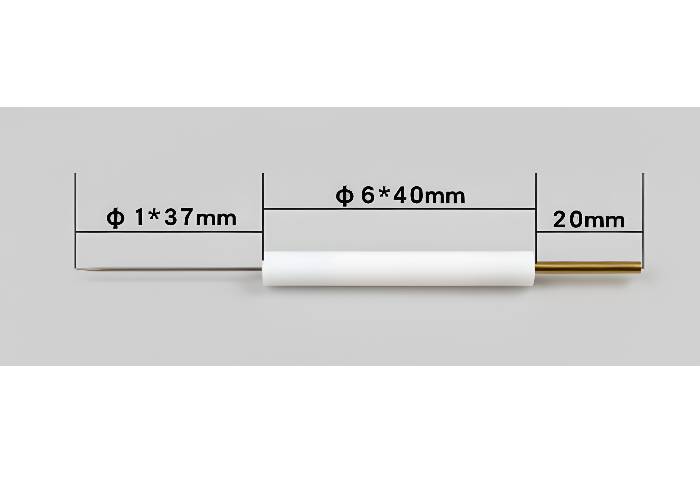
Physical Specifications
-
Tube diameter: 6-12 mm (varies by model)
-
Effective length: 70-150 mm
-
Filling solution: Saturated K2SO4 or defined H2SO4
-
Potential stability: <5 mV
-
Operating temperature: 0-40°C (standard models) up to 100°C (specialized versions)
-
Junction type: Porous ceramic frit or electroporous KT glass
Key Performance Advantages
Superior Chemical Robustness
The Hg/Hg2SO4 reference electrode excels in chloride-rich environments where traditional Ag/AgCl electrodes may experience performance degradation. Its resistance to chloride poisoning makes it the preferred choice for corrosion testing, electroplating studies, and lead-acid battery applications.
Exceptional Stability & Low Drift
This reference system delivers outstanding potential stability with minimal drift over extended measurement periods. The electrode recovers quickly from small temperature excursions, maintaining measurement accuracy even under challenging operational conditions.
Optimized Junction Performance
The porous frit junction design provides steady electrolyte flow while minimizing liquid junction potentials. This configuration ensures consistent ionic conductivity and reduces measurement uncertainty across various experimental conditions.
Primary Applications
Corrosion Testing & Analysis
The electrode serves as the industry standard for corrosion studies in chloride-containing media, providing reliable reference potentials for materials evaluation, protective coating assessment, and infrastructure monitoring applications.
Lead-Acid Battery Research
Battery testing and maintenance represent major application areas, particularly for measuring half-cell potentials in lead-acid systems. The electrode's compatibility with sulfuric acid electrolytes makes it indispensable for battery performance evaluation and cycle life studies.
Electrochemical Analysis
Research applications include electroanalysis, potentiometric titrations, and fundamental electrochemistry where mercury handling protocols are acceptable and chloride contamination must be avoided.
Acidic Electrodeposition
The electrode proves essential for electroplating and electrodeposition studies in acidic media, enabling precise potential control during metal deposition processes while avoiding chloride interference.
Operational Considerations
Maintenance Requirements
Proper electrode care involves maintaining saturated sulfate fill levels, preventing bubble formation in the electrolyte, and periodic verification against standard references. The filling solution should retain a few crystals to ensure sulfate saturation.
Safety & Handling
Mercury handling and disposal procedures apply to all operations involving this electrode type. Users must consult institutional safety policies and follow established protocols for mercury-containing equipment.
Temperature Management
Due to the electrode's negative temperature coefficient and thermal hysteresis, temperature fluctuations should be minimized during measurements. Advanced configurations may include extended salt bridge tubes to isolate the electrode from temperature variations.
Comparative Advantages
Versus Ag/AgCl Electrodes
The Hg/Hg2SO4 system offers superior chloride resistance and maintains stability in sulfate-rich environments where Ag/AgCl electrodes may experience contamination issues.
Versus Calomel Electrodes
Compared to saturated calomel electrodes, the Hg/Hg2SO4 provides enhanced stability in sulfate-containing solutions and avoids chloride-related complications in sensitive analytical procedures.
Environmental Compatibility
The electrode's sulfate-based internal solution makes it ideally suited for applications in sulfuric acid media, lead-acid battery systems, and other sulfate-containing environments where competing reference electrodes may prove inadequate.
Quality Assurance & Standards
Science Gears' Hg/Hg2SO4 reference electrodes are manufactured using reagent-grade mercury sulfate and high-purity electrolyte solutions. Each electrode undergoes rigorous quality testing to ensure potential stability, junction integrity, and long-term reliability. The electrodes feature traceable identification and include comprehensive documentation with safety data sheets.
The electrode represents an essential tool for researchers and analysts requiring dependable reference potentials in challenging electrochemical environments where chloride exclusion and exceptional stability are paramount considerations
Resources & Downloads
Everything you need to get started
Reference Electrode FAQ & Maintenance Guide
Customer Reviews
Related Product
Explore our precision instruments designed for electrochemical research and energy applications
Still Wondering About Something?
Explore our FAQ for fast, clear answers to the most common questions—available 24/7.