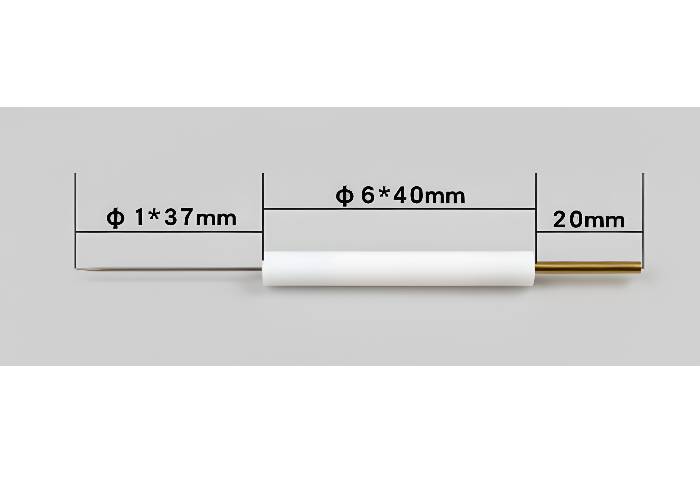
Non-aqueous Ag/Ag+ Reference Electrode
Non-Aqueous Ag/Ag⁺ Reference Electrode (Ag/AgNO₃) for organic electrochemistry
Product Description
The Non-Aqueous Ag/Ag⁺ Reference Electrode (often referred to as an Ag/AgNO₃ reference electrode) is designed for non-aqueous electrochemistry, where conventional aqueous reference electrodes (such as Ag/AgCl and SCE) are often unsuitable due to solvent incompatibility, moisture sensitivity, and unstable liquid junction potentials. It supports more reproducible potential control for organic electrochemistry, including cyclic voltammetry (CV), pulse methods, electrocatalysis screening, and molecular redox studies in non-aqueous media.
In a typical non-aqueous Ag/Ag⁺ reference system, a silver element is equilibrated with Ag⁺ ions supplied by silver nitrate (AgNO₃) dissolved in a compatible organic solvent (commonly acetonitrile-based systems in published practice). For improved stability and conductivity, the filling solution is commonly prepared with a supporting electrolyte (for example, tetrabutylammonium salts such as TBAPF₆), selected to suit the electrolyte system used in your cell.
Why both AgNO₃ and a supporting electrolyte are used
A reference electrode must contain a well-defined redox couple to set a stable potential. AgNO₃ supplies Ag⁺, enabling the defining equilibrium Ag ⇌ Ag⁺ + e⁻, which sets the reference potential. A supporting electrolyte (for example, TBAPF₆) does not define the reference potential on its own, but it is important because it improves ionic conductivity and helps maintain a more stable liquid junction potential at the frit. In practice, using AgNO₃ + supporting electrolyte together helps reduce noise and drift and improves repeatability in non-aqueous measurements.
Typical filling solution guidance (common laboratory practice)
- AgNO₃ concentration: 0.01–0.1 mol/L
- Supporting electrolyte (example: TBAPF₆): often prepared at ~0.1 mol/L, consistent with the experimental electrolyte system where suitable
- Reporting best practice: use an internal standard such as ferrocene/ferrocenium (Fc/Fc⁺) for inter-laboratory comparability
Typical applications
- Non-aqueous cyclic voltammetry (CV) in organic solvents
- Molecular electrochemistry and redox potential studies
- Electrocatalysis screening in moisture-sensitive electrolytes
- Organic electrosynthesis and mechanistic electrochemistry
- Spectroelectrochemistry where aqueous references are unsuitable
Related technical guide (internal link)
For a practical selection guide and reporting recommendations, see:
How to Choose the Right Reference Electrodes
https://www.sciencegears.com.au/blogs/how-to-choose-the-right-reference-electrodes
Note: Always confirm solvent and electrolyte compatibility for your specific experimental conditions.
Resources & Downloads
Everything you need to get started
Reference Electrode FAQ & Maintenance Guide
Customer Reviews
Related Product
Explore our precision instruments designed for electrochemical research and energy applications
Still Wondering About Something?
Explore our FAQ for fast, clear answers to the most common questions—available 24/7.