Electrochemistry Accessories
High-quality electrodes, electrochemical cells, clamps, and holders for optimising lab experiments. Ideal for battery, corrosion, sensor, and EIS research.

Working Electrode

Reference Electrodes

Counter Electrodes

Electrode Clamp/Holder

Metal Foam Electrodes

Metal Mesh Electrodes

Microelectrodes

Electrochemical Cell

H-Cell

Membrane Electrode Assembly (MEA) Test Cells

Ion-Exchange Membranes

Corrosion Test Electrochemical cell
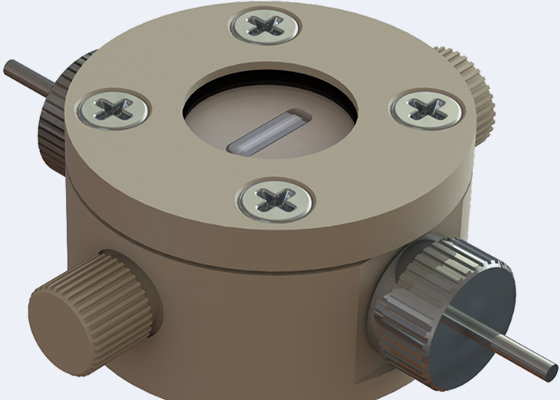
Battery Test Cell options
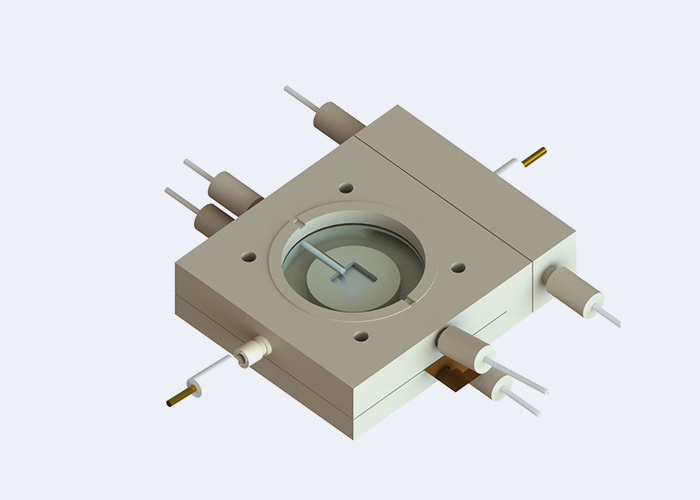
In-Situ & Operando Electrochemical Cells

Photoelectrochemical Cells

RDE/RRDE Electrodes
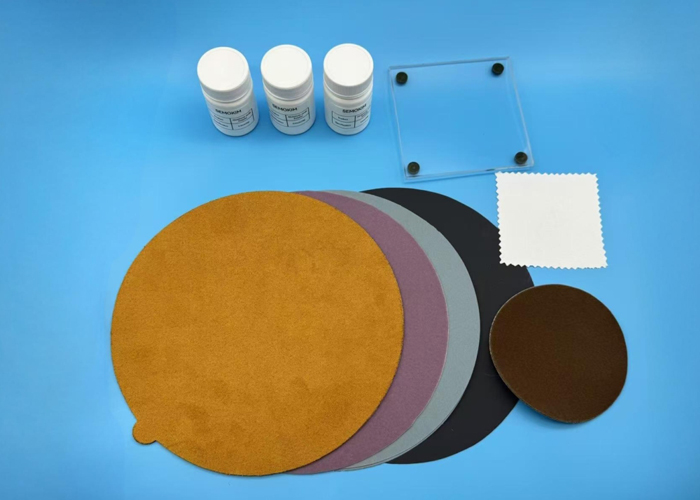
Other Electrochemistry Accessories
Electrochemistry Accessories
Electrochemistry accessories are critical components that enable accurate, reliable, and reproducible electrochemical experiments. These tools are foundational to research in energy storage, corrosion science, electrochemical sensing, and materials analysis. From precision electrodes to specialised electrochemical cells and electromagnetic shielding, each accessory contributes to the control, measurement, and customisation of electrochemical systems.
What Are Electrochemistry Accessories?
Electrochemistry accessories refer to a broad range of specialised instruments and components that support electrochemical measurements. These include electrodes (working, reference, and counter), electrochemical cells, electrode clamps and holders, polishing kits, and Faraday cages. Each component plays a unique role in improving data precision, experimental repeatability, and setup flexibility across various research environments.
Core Electrochemistry Accessories
1. Working Electrodes
The working electrode (WE) is where the target redox reaction occurs. Its material and geometry directly affect the experiment's sensitivity, selectivity, and response kinetics. Selection depends on the analyte, electrolyte, and experimental objective.
Common types:
- Carbon Paste Electrode – easily renewable surface for adsorptive studies.
- Disc Electrodes – precise geometry for reproducible surface area.
- L-Type Working Electrodes – convenient geometry for vertical cells.
- Plate Electrodes – large surface area for high current experiments.
- Rod Electrodes – a robust choice for general applications.
2. Reference Electrodes
Reference electrodes provide a stable and well-defined potential for accurate measurement of the working electrode's voltage. They are chemically isolated and selected based on the electrolyte composition (aqueous or non-aqueous), pH, and temperature range.
Examples include:
- Ag/AgCl Reference Electrode – standard for aqueous systems.
- Non-aqueous Ag/Ag⁺ Reference Electrode – for organic solvents.
- Saturated Calomel Electrode (SCE) – widely used, highly stable.
- Double Salt Bridge SCE – minimises junction potential.
- Hg/Hg₂SO₄ and Hg/HgO Electrodes – for alkaline or sulphate electrolytes.
- Reversible Hydrogen Electrode (RHE) – used in hydrogen evolution studies.
3. Counter Electrodes
Counter electrodes (CEs) complete the circuit by carrying the current that balances the working electrode reaction. They are typically chemically inert and electrochemically stable to avoid unwanted side reactions.
Common types:
- Platinum Wire Electrode / Helix / Mesh / Plate / Gauze – ideal for high-purity experiments.
- Graphite Rod Counter Electrode – economical, inert, and suitable for many applications.
- Platinum Electrode Clamp – for securely holding thin Pt wires or foils.
4. Electrode Clamps and Holders
Clamps and holders ensure stable electrical contact and proper electrode positioning within the cell. High-quality clamps prevent contamination and maintain consistency in measurements.
Variants include:
- Platinum Electrode Clamp
- Gold Electrode Clamp
- Glassy Carbon Electrode Clamp
- Graphite Electrode Clamp
- Stainless Steel Electrode Clamp
5. Electrochemical Cells
Electrochemical cells provide the reaction environment where electrodes and electrolytes interact. Their design and material compatibility are critical for experiment reproducibility.
Types of electrochemical cells:
- Analytical Cells – standard setup with provisions for gas purging and temperature control.
- H-Cells – dual compartments for separating anodic/cathodic products; ideal for redox and electrolysis studies.
- Corrosion Test Cells – including Avesta and flat cells- enable crevice, pitting, or uniform corrosion testing.
- Battery Test Cells – multi-position or coin cell holders for evaluating battery charge/discharge performance.
- Spectroelectrochemical Cell & Photoelectrochemical Cells – designed for simultaneous optical and electrochemical monitoring.
6. Rotating Electrodes (RDE and RRDE)
Rotating Disk Electrodes (RDEs) and Rotating Ring-Disk Electrodes (RRDEs) are used to control mass transport during electrochemical reactions, which are essential for studying reaction kinetics and mechanistic pathways.
Examples:
- GC Disk Electrode (RDE) – for controlled diffusion experiments.
- Pt Ring-RRDE Electrode – enables detection of intermediate species at the ring.
7. Electrode Polishing Kits
Polishing kits are essential for surface preparation of working electrodes. A smooth, clean electrode surface ensures reproducibility, minimises noise, and enhances signal clarity, especially in voltammetry.
Includes:
- Polishing cloths
- Abrasive compounds
- Holders for polishing pads
8. Faraday Cage
A Faraday cage is a metal enclosure that shields sensitive electrochemical systems from electromagnetic interference (EMI). This is crucial when working with high-impedance cells, nanoampere-level currents, or potentiostats operating at low signal levels. It helps eliminate external noise and improve data integrity.
Summary
Electrochemistry accessories are not just add ons they are the enabling components of every successful electrochemical experiment. Whether you're characterising battery materials, studying corrosion mechanisms, or measuring photocurrents, the right accessory ensures accuracy, reproducibility, and confidence in your data.
FAQs
1. Customisation and Support
ScienceGears partners with leading manufacturers to offer custom electrodes, cell designs, and modular setups tailored to your experiment. We provide expert consultation to help select or design accessories that best match your material systems, electrolyte environment, and research goals.
2. Why Are Electrochemistry Accessories Important?
Electrochemical experiments require tight control over variables, and accessories provide the hardware foundation for that control. From reliable reference electrodes and optimised cells to electromagnetic shielding, these tools determine whether your results are reproducible and scientifically valid. For any lab working on batteries, fuel cells, corrosion, electrosynthesis, or sensors, the right accessories are as critical as the instrument itself.
3. How to Choose the Right Accessories?
When selecting accessories, consider the following:
- Type of Experiment – corrosion, analytical, battery, or optical-electrochemical studies.
- Electrolyte Compatibility – aqueous vs. non-aqueous systems.
- Sensitivity Requirements – low-noise setups benefit from Faraday cages and well-polished electrodes.
- Environmental Conditions – temperature, gas atmosphere, and pH impact accessory selection.
4. What is a working electrode, and how do I choose one?
The working electrode is where redox reactions take place and signals are generated in response to the electrochemical process. Its material (e.g., glassy carbon, platinum, gold) and shape (disc, plate, rod, etc.) influence reaction kinetics, signal stability, and sensitivity. Choosing the right working electrode depends on the analyte, solvent system, and experimental goals.
5. What accessories are most important for corrosion studies?
Corrosion studies require accessories that simulate real-world conditions such as crevice geometry or chloride exposure. Avesta and flat corrosion test cells are commonly used, along with appropriate reference electrodes and inert counter electrodes. Environmental controls like temperature and oxygen removal enhance result accuracy.
6. How can I minimize noise in my electrochemical measurements?
Noise in electrochemical measurements can arise from electromagnetic interference, ground loops, or unstable reference electrodes. A Faraday cage, proper cable shielding, and clean contact surfaces can significantly reduce this noise. Ensuring stable environmental conditions and proper equipment calibration also helps improve data quality.
7. What is the difference between RDE and RRDE?
Rotating Ring-Disk Electrodes (RRDE) have a disk in the center and a concentric ring electrode, allowing simultaneous measurement of intermediate or secondary reaction products. This configuration is useful for mechanistic studies where products generated at the disk are detected at the ring. Applications include oxygen reduction reaction (ORR) studies and multi-step electrochemical reactions.
8. How does a Faraday cage improve electrochemical measurements?
A Faraday cage shields sensitive electrochemical setups from external electromagnetic interference (EMI), which can distort low-current signals. It acts as a grounded metal enclosure around the cell and cables, providing a noise-free environment. This is particularly important in high-impedance measurements or when working with nanoampere-level currents.
