Disc Working Electrodes (Glassy Carbon, Noble Metals & Metal Discs)
Disc Working Electrodes (Glassy Carbon, Noble Metals & Metal Discs)
Disc working electrodes for controlled, reproducible electrochemistry
Disc electrodes are a foundational accessory in electrochemistry because they provide a well-defined, polishable geometric area for the working electrode. A circular disc of conductive material is embedded into an insulating body so only the flat face contacts the electrolyte. This design helps reduce edge leakage currents, improves reproducibility, and makes it easier to compare current density, kinetics, and surface effects across experiments.
In a standard three-electrode setup, the disc electrode is where electron-transfer reactions occur while the potentiostat controls potential/current relative to a reference electrode and completes the circuit via a counter electrode. A refreshed, mirror-polished surface typically improves signal stability, lowers background noise, and reduces artefacts caused by fouling or oxide films.
What ScienceGears supplies (materials and diameters)
ScienceGears offers a wide selection of disc working electrode materials to match your electrolyte chemistry, potential window, catalysis needs, and surface functionalisation workflows:
- Glassy Carbon: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11.28, 12, 15, 20 mm
- Gold: 0.5, 1, 2, 3, 4, 5, 6 mm
- Platinum: 0.5, 1, 2, 3, 4, 5, 6 mm
- Silver: 2, 3, 4, 5 mm
- Copper: 2, 3 mm
- Titanium: 2, 3 mm
- Aluminium: 2, 3 mm
- Nickel: 2, 3 mm
- Palladium: 1, 2, 3, 4, 5, 6 mm
- Cobalt: 3, 4, 5, 6 mm
- Zinc: 2, 3 mm
- Tungsten: 2 mm
- Lead: 2 mm
- Cadmium: 2, 4 mm
- Iron: 2 mm
- Indium: 2 mm
- Stainless Steel: 5 mm
- Carbon Steel: 5 mm
Typical construction (useful for method planning)
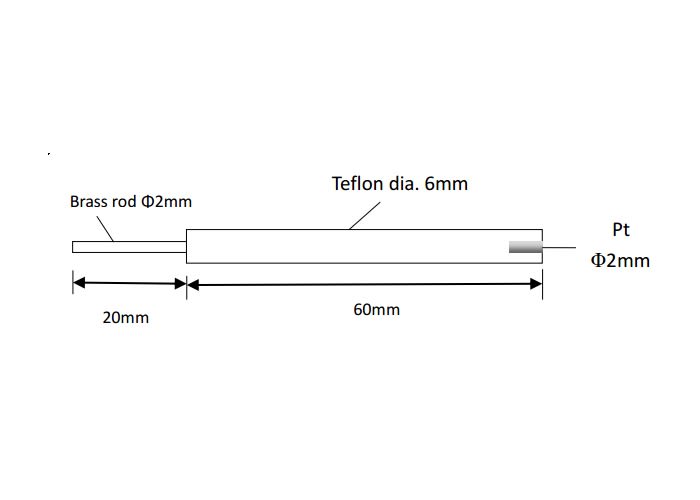
- Coat / insulator: PTFE (chemical resistance and electrical insulation)
- Brass rod length: ~20 mm
- Jacket length: ~60–100 mm
- Total length: ~80–120 mm
This geometry is designed for straightforward integration with common electrode holders and benchtop cell setups.
Custom disc electrodes (on request)
If you need a disc electrode not shown above, ScienceGears can often arrange custom disc electrodes on request. This may include alternative materials/alloys, non-standard diameters, different embedding/insulator materials, or modified lengths to suit specific cell geometries and electrode holders. Share your target material, diameter, electrolyte/solvent, and connection or mounting requirements, and we’ll advise on a suitable configuration and feasibility.
How to choose the right disc electrode
Choose material based on electrochemical behaviour and surface chemistry:
- Glassy carbon: broad usability, low background, great for general voltammetry, sensors, and organic/aqueous electrolytes
- Platinum / palladium: strong electrocatalytic activity (useful for hydrogen/oxygen reactions and mechanistic studies)
- Gold: surface functionalisation (thiols/self-assembled monolayers), adsorption studies, and catalysis/sensing applications
- Base metals/alloys (Ni, Cu, Co, Fe, Ti, stainless, carbon steel, etc.): corrosion, passivation, plating, and material-specific electrocatalysis
Choose diameter based on signal level and mass transport:
- Smaller discs can help reduce total current (useful for low-volume cells or high-resistance electrolytes).
- Larger discs increase signal and allow higher-current experiments (useful for electrolysis screening or when higher current density is required).
Typical techniques and applications
- Voltammetry: CV, LSV, DPV, SWV (mechanism, kinetics, analytical chemistry)
- EIS: charge-transfer resistance, double-layer capacitance, coating performance
- Electrocatalysis: HER/OER/ORR, CO₂ reduction, fuel cell catalyst screening
- Corrosion & materials: passivation films, inhibitor studies, surface treatment comparisons
- Electrodeposition: plating/stripping, nucleation and growth studies
Why researchers choose ScienceGears
ScienceGears supports AU/NZ researchers with a broad, practical selection of electrode materials and diameters and compatibility-first guidance, including pairing with:
- Potentiostats / galvanostats for CV/EIS and advanced methods
- Electrochemical cells (standard, gas-tight, low-volume, jacketed formats)
- RDE/RRDE workflows where controlled hydrodynamics and product detection are required
- Polishing and electrode accessories to maintain reliable surface condition over time
FAQs
1) What is a disc working electrode used for in electrochemistry?
A disc working electrode provides a defined circular area where reactions occur under potentiostat control. It is widely used for voltammetry and EIS because it can be polished to a repeatable surface condition.
2) Why does electrode diameter matter?
Diameter sets the geometric area, which directly affects measured current and current density. Larger discs deliver higher total currents; smaller discs can improve control in low-volume cells or high-resistance electrolytes.
3) Which disc electrode material should I choose: glassy carbon, gold, or platinum?
- Glassy carbon suits general-purpose voltammetry with low background currents.
- Gold is often chosen for surface functionalisation and adsorption studies.
- Platinum is common for catalysis and robust electrochemical cycling.
4) Can I use metal disc electrodes for corrosion testing?
Yes. Stainless steel, carbon steel, nickel, copper, titanium and other metals are frequently used for corrosion and passivation studies when you need material-relevant electrochemical behaviour.
5) How do I clean or refresh a disc electrode surface?
Most disc electrodes are refreshed by mechanical polishing (appropriate polishing pads/films and alumina or diamond slurry depending on material), followed by rinsing and, if needed, brief electrochemical cleaning cycles.
6) What causes noisy or drifting voltammograms with disc electrodes?
Common causes include incomplete polishing, residual slurry contamination, surface oxides (especially on reactive metals), bubbles on the electrode face, poor electrical contact, or unstable reference electrode placement.
7) Are these disc electrodes compatible with my potentiostat?
In most cases yes, provided you have a compatible electrode holder/lead connection for the electrode body. If you’re using specialised setups (low-volume caps, gas-tight lids, or rotators), match the mechanical format and connector style.
8) When should I use an RDE/RRDE instead of a stationary disc electrode?
Use RDE/RRDE when you need controlled hydrodynamics to separate kinetics from mass transport, or when you want to detect intermediates/products at a ring electrode (RRDE) for mechanistic insight.
9) Can I run non-aqueous electrolytes with these electrodes?
Many labs do. Material choice and cleaning become more critical, and solvent compatibility depends on the electrode body/insulator (PTFE is commonly selected for chemical resistance).
10) What related products should I consider with disc electrodes?
Most researchers also use: a reliable reference electrode, a suitable counter electrode, an appropriate electrochemical cell, and a stable potentiostat (plus polishing consumables for repeatability).
For further information or to discuss your specific research requirements, please contact us.
Technical Specifications
Precision engineering for accurate measurements
| Electrode Material | Available Diameter (mm) |
|---|---|
| Glassy Carbon | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11.28, 12, 15 and 20 |
| Gold | 0.5, 1, 2, 3, 4, 5 and 6 |
| Platinum | 0.5, 1, 2, 3, 4, 5 and 6 |
| Silver | 2, 3, 4 and 5 |
| Copper | 2 and 3 |
| Titanium | 2 and 3 |
| Aluminium | 2 and 3 |
| Nickel | 2 and 3 |
| Palladium | 1, 2, 3, 4, 5 and 6 |
| Cobalt | 3, 4, 5 and 6 |
| Zinc | 2 and 3 |
| Tungsten | 2 |
| Lead | 2 |
| Cadmium | 2 and 4 |
| Iron | 2 |
| Indium | 2 |
| Stainless Steel | 5 |
| Carbon Steel | 5 |
Methods/Techniques
Want to learn more about these techniques?
Check out our Introduction to Electrochemical Techniques blog for an in-depth overview of their principles and applications.
Customer Reviews
Related Product
Explore our precision instruments designed for electrochemical research and energy applications

Carbon Paste Electrode
- Electrode Material: Carbon Paste
- Available Diameter (mm): 2, 3 and 5

L-Type Working Electrodes
- Electrode Material: Available Diameter (mm)
- Glassy Carbon: 2, 3 and 5
- Gold: 2, 3 and 5

Plate Electrodes
- Electrode Material: Available Diameter (mm)
- Glassy Carbon: 10 x 10 x 2
- Glassy Carbon: 10 x 10 x 2
- Platinum: 10×10×0.1

Rod Electrodes
- Electrode Material: Available Diameter (mm)
- Glassy Carbon: 3, 4, 5 and 6
- Graphite: 3, 4, 5 and 6
Still Wondering About Something?
Explore our FAQ for fast, clear answers to the most common questions—available 24/7.